The new form Manufacturer Investigation form for reportable events in EU is mandatory starting this…
What EU is doing better than the US: Closed Loop Post Market Surveillance “CLPS”

With MDR implementation, companies are linking risk management and complaint systems and post market surveillance system. Many US companies were already doing this because FDA inspections check to see if complaints, CAPA, and risk analysis are linked.
“Product and quality problems should be analyzed to identify product and quality problems that may require corrective action. The firm should routinely analyze quality data regarding product and quality problems. This analysis should include data and information from all acceptance activities, complaints, service, and returned product records.” (Reference Pg 51 QSIT Guide)
MDR added annual reports (PSUR and SSCP) that included review of CAPA, previous annual reports (PSUR), and serious complaints. Adding transparency, the MDR mandated the annual reports needed to be made available to the public.
Closed Loop Post Market Surveillance “CPLS”
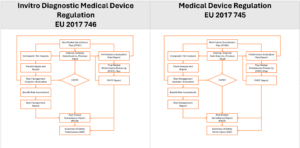
This builds on the management review and expectation of management control. Over time, the effectiveness of management controls will be publicly visible. MDR enforcement is behind auditing enforcement but is ahead of making management responsible and transparent to the public. In contrast, US unannounced inspections and published findings are usually after issues have occurred.
Compliance enforcement of MDR has been delayed, but company compliance is more visible for consumers to be informed on management commitment to quality improvements.
Time will tell if people connect the dots, but baseline data of compliance performance is being published of medical device manufacturers marketing products in the EU market.
With the new reporting requirements, EU is making a difference in data relevancy, transparency, and approach in regards to establishing a closed loop post market surveillance system.
Where to start?
Helpful Documents:
Post Market Surveillance Procedure-MDR – QMS Velocity


